We’ve all heard about the benefits of recycling – and it turns out it’s good for your body too! Autophagy is often described as the body’s recycling process and is linked with reduced risk of many diseases as well as longer lifespan. It’s a hot topic in medical research and may help prevent and even cure many serious health disorders. But what is autophagy? And how can we benefit from it? In this post we answer these questions and take a look at the health benefits of autophagy.
Contents:
- What is autophagy?
- Autophagy and longevity
- Autophagy and cancer
- Autophagy and Alzheimer’s
- Autophagy and diabetes
- Autophagy and inflammation
- Autophagy and infectious diseases
- How to induce autophagy
- Summary
What is autophagy?
The word autophagy roughly translates as ‘self-eating’ – and that’s pretty much what happens at a cellular level.
Put simply, autophagy can be thought of as the body’s recycling process. Components of cells that no longer work or aren’t needed get broken down during autophagy and used elsewhere where they are more useful.
The main form of autophagy is macroautophagy. During this process, the cell identifies the parts it no longer requires and wraps them into an autophagosome. To continue the recycling analogy, the autophagosome can be thought of as a cellular garbage bag.
This garbage bag is then transported to a lysosome where the contents are broken down by acidic hydrolases. These constituent parts can then be used elsewhere.
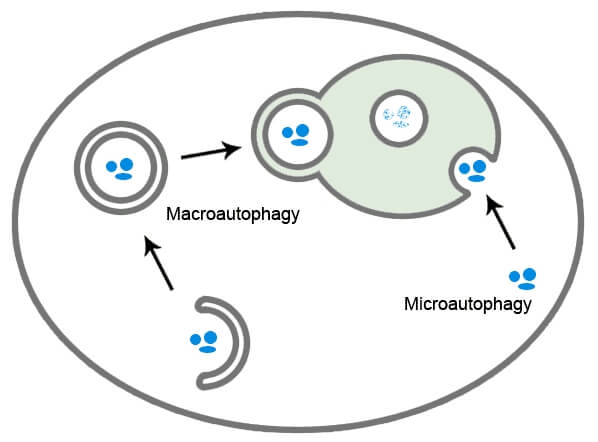
At a cellular level, autophagy prevents the build up of toxic waste products, helps fight disease by removing invading pathogens and in cases of nutrient starvation promotes cellular survival by maintaining energy levels.
And at the level of the organism, autophagy is associated with a whole host of huge health benefits.
Autophagy and longevity
“Both starvation and the genetic inactivation of nutrient signaling converge on the induction of autophagy, a cytoplasmic recycling process that counteracts the age-associated accumulation of damaged organelles and proteins as it improves the metabolic fitness of cells […] mounting evidence suggest[s] that autophagy is not only necessary but, at least in some cases, also sufficient for increasing longevity.“
Essential role for autophagy in life span extension. PMCID: PMC4382258
When it comes to health benefits there’s few more desirable than living better and living longer. Autophagy may help achieve this.
It’s long been known that caloric restriction extends lifespan. This is true in mice1, in worms1, in yeast1 – just about every organism that’s been tested.
And the reason for this may be due, at least in part, to autophagy.
The authors of this paper describe how autophagy counteracts a key feature of ageing: the accumulation of damaged organelles and proteins. As we age, these damaged cellular components build up and reduce the metabolic fitness of cells. Autophagy clears up these damaged components and in turn improves metabolic efficiency within the cell.
Autophagy can also remove damaged mitochondria – the part of the cell used to create energy.
Damaged mitochondria can produce free radicals and increase oxidative stress. You’ve heard of the health benefits of antioxidants – well this is basically the opposite effect of that1. But by removing these damaged mitochondria, autophagy eliminates this oxidative stress and protects against these harmful effects.
These mechanisms are associated with longer lifespan as well as delayed or reduced risk of age-related diseases.
Autophagy and cancer
“By preventing the toxic accumulation of damaged protein and organelles, particularly mitochondria, autophagy limits oxidative stress, chronic tissue damage, and oncogenic signaling, which suppresses cancer initiation.“
Autophagy, Metabolism, and Cancer. PMCID: PMC4646728
The same mechanisms described above – clearing out cellular garbage, reduced oxidative stress, etc. – also reduce the risk of cancer.
And the cancer fighting benefits of autophagy are further bolstered by the fact that many anti-cancer drugs also induce this process. Chemotherapy and radiation, for example, both induce autophagy within cancerous cells1.
What’s more, this study reports that breast cancer cells have decreased expression of genes related to autophagy (beclin 1). This suggests that autophagy may help fight the development and progression of breast cancer.
Similarly, ovarian cancer grows less aggressively and responds more favorably to chemotherapy when this autophagy gene is upregulated1.
But it’s not as simple as autophagy = less cancer.
The link between autophagy and cancer is not yet fully understood. Evidence is conflicting. While some studies suggest autophagy protects against cancer, others suggest this process can be used by tumor cells themselves to proliferate – especially during the later stages1.
Given that cancer represents over 200 different diseases1, and the wide-ranging effects of autophagy throughout the body, it’s perhaps unsurprising that the exact relationships are still being uncovered.
Nevertheless, autophagy-related therapies represent a new and exciting avenue for cancer prevention and treatment.
Autophagy and Alzheimer’s
“There is emerging agreement that defects in autophagy are likely to contribute to the neurodegenerative processes in numerous diseases, including Alzheimer’s disease (AD). […] it appears evident that therapeutic strategies that enhance autophagy have the potential to be beneficial in AD.“
Autophagy in Alzheimer’s Disease. PMCID: PMC5039008
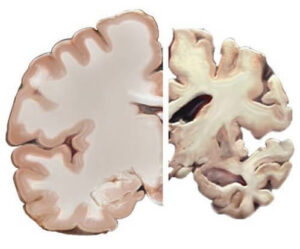
Alzheimer’s disease is the most common form of dementia. It’s a progressive disorder, meaning symptoms – memory loss, mood swings and difficulty thinking and speaking – get worse over time. At present, there is no cure.
The brains of individuals with Alzheimer’s develop a buildup of senile plaques. These are composed of amyloid β (Aβ), and neurofibrillary tangles (NFTs) which are made up of tau protein1. Studies have shown individuals with Alzheimer’s have diminished ability to clear Aβ compared to healthy brains1.
But autophagy greatly influences the metabolism of both tau protein and Aβ1. It helps break down Aβ and clear it out, preventing the buildup of amyloid plaques.
This link is demonstrated in this study from 2013. Here, mice with genes for impaired autophagy were unable to secrete Aβ, resulting in an accumulation within brain cells. This buildup of Aβ was thought to be responsible for neurodegeneration and memory impairment similar to symptoms of Alzheimer’s.
Inflammation is another mechanism implicated in Alzheimer’s disease1 – and one that is also reduced via autophagy (see below).
And research suggests autophagy may also be beneficial for other neurodegenerative disorders such as Huntington’s disease1 and Parkinson’s1.
Autophagy and diabetes
“An emerging body of evidence supports a role for autophagy in the pathophysiology of type 1 and type 2 diabetes mellitus.”
The emerging role of autophagy in the pathophysiology of diabetes mellitus. PMCID: PMC3359481
Diabetes mellitus is the name for a group of metabolic disorders associated with sustained high blood sugar levels.
Type 1 diabetes is caused by an inability of the pancreas to produce insulin – the hormone that lowers blood sugar.
With type 2 diabetes, the pancreas often produces adequate insulin but the cells do not respond in the usual way. Rather than absorbing the sugar, cells become resistant and so the sugar remains in the blood.
And autophagy may play a role in both type 1 and type 2 versions of the disorder.
This paper describes how autophagy protects against the effects of reactive oxygen species. Mitochondria (the part of the cell that produces energy) release free radicals as part of their normal function. As described earlier, autophagy helps clear up these reactive oxygen species – effectively acting as an antioxidant.
However, in cases of type 2 diabetes, these antioxidant effects are impaired. This causes reactive oxygen species to build up in the cell. This impairs mitochondrial function – a mechanism associated with insulin resistance.
It follows from this that increased autophagy would help clear out reactive oxygen species, improve mitochondrial function and avoid insulin resistance.
The same paper also describes how autophagy may impact the development of type 1 diabetes. Type 1 diabetes is caused by destruction of insulin producing pancreatic beta cells. And since autophagy is known to regulate apoptosis and necrosis (cell death), the authors speculate that impaired autophagy may play a role in the development of type 1 diabetes too.
Autophagy and inflammation
When your body is damaged or under attack – say from infection or an injury – a little bit of inflammation is necessary to remove the threat and repair the damage. This kind of acute inflammation tends to go down after a few days once the body is back to normal. It’s a good thing.
But there’s another kind of inflammation: chronic. And it’s thought to play a vital role in many modern health issues, including:
- Cardiovascular disease1,2,3,4
- Cancer1,2,3
- Type 2 diabetes1,2,3
- Alzheimer’s disease1,2,3
- Depression1,2
Chronic inflammation occurs when the body is unable to clear the problem. If left unchecked, it can wreak havoc in the body and lead to the kinds of mental and physical disorders listed above.
Because of these devastating effects, inflammation is a huge topic in health at the moment.
It’s thought that the modern, ‘Western’, diet is a significant contributing factor. Processed foods, refined sugars, an excess of omega 6 fatty acids relative to omega 3; these can all create a state of chronic inflammation within the body.
Besides medication, there are a number of ways to control this inflammation. The most obvious is to address any dietary or lifestyle factors that exacerbate it.
Another way may be via autophagy. Recent studies have highlighted the role autophagy plays in regulating inflammation.
The effects of autophagy can be both pro-inflammatory and anti-inflammatory.
This study, for example, describes how autophagy initially increases inflammation by promoting the development and survival of inflammatory cells such as macrophages and lymphocytes.
Autophagy then reduces inflammation by clearing out the pro-inflammatory molecules and antigens that get released in response to invasion1.
Defects in autophagy-related genes have been identified as risk factors for inflammatory diseases such as cystic fibrosis1 and Crohn’s disease1 – raising the possibility of related treatments in future.
What’s more, defective autophagy looks likely to play a role in inflammation-related metabolic diseases such as obesity and diabetes due to its effects on endoplasmic reticulum stress and insulin resistance1.
But as the authors of this paper note, however, our current understanding of the exact relationship between autophagy and inflammation is still fairly primitive.
Autophagy and infectious diseases
The mechanisms of autophagy also help fight infection and may even pave the way to cure infectious diseases such as HIV, tuberculosis and streptococcal infections.
This paper describes at least three ways autophagy fights infection.
Firstly, autophagy is known to moderate immune response by helping the body recognize pathogens and endogenous sources of inflammation.
Secondly, autophagy enhances the removal of toxins associated with infection such as Staphylococcus aureus α-toxin1.
But most importantly, autophagy directly eliminates microbes such as invading bacteria or viruses in a process known as xenophagy.
The authors go on to describe how autophagy – either conventional autophagy or a modified form – could potentially be harnessed to target viruses such as HIV.
And the potential to fight and possibly eliminate HIV using autophagy has been described in at least one other paper:
“This study is the first to demonstrate that selective autophagy can be an antiviral process by degrading a viral transactivator. In addition, the results could help in the design of new therapies against HIV-1 by specifically targeting this mechanism.“
Further research suggests these same mechanisms could be used to tackle other infectious diseases such as tuberculosis1 and streptococcal infections1 as well.
How to induce autophagy
There are a number of ways to stimulate or increase autophagy within the body and start reaping these health benefits. These include:
Fasting and calorie restriction
Autophagy may be an evolutionary adaptation to increase cellular energy in times where nutrition is restricted.
So it’s perhaps unsurprising that restricting caloric intake – whether through calorie restriction or fasting – is an effective way to induce autophagy.
Fasting (going without food) is quickly being recognized for its health benefits beyond simply weight loss. There are two main kinds of fasting: prolonged and intermittent.
Intermittent fasting typically consists of restricting food intake to a set number of hours per day. For example, you may consume all your calories within an 8 hour window and fast for the other 16. Some individuals may opt for an even smaller eating window – 18:6 or 20:4, say – to increase the benefits.
Prolonged fasting is a little more extreme. It consists of forgoing food altogether for an extended period of time – sometimes for days or even weeks.
But both intermittent and prolonged fasting can induce autophagy1,2,3.
“[Prolonged fasting (PF)] cycles lasting 2 or more days but separated by at least a week of a normal diet are emerging as a highly effective strategy to protect normal cells and organs from a variety of toxins and toxic conditions […] PF causes a decrease in blood glucose, insulin and insulin-like growth factor I (IGF-I) […] and is accompanied by autophagy“
The study above tested the effects of a diet that mimics fasting on mice. The researchers observed many of the health benefits described elsewhere in this article – reduced incidence of cancer, improved cognitive performance, increased longevity – in the mice as well as an increase in autophagy.
Other studies in mice report increased autophagy as a result of intermeal fasting – an eating schedule similar to intermittent fasting1.
Ketogenic diet
A ketogenic diet is a high fat, low carbohydrate diet.
The lack of sugar and carbohydrates on a ketogenic diet causes glucose levels in the body to drop. Eventually, this causes the body to stop relying on glucose for energy and instead create fatty acids and ketone bodies from fat. These can then be used for energy instead.
Historically, ketogenic diets been used to treat epilepsy – with surprising efficacy. Research is now emerging that it may also be an effective way to prevent and treat many of the health disorders discussed in this article: Alzheimer’s disease, Parkinson’s disease, cancer and so on1,2.
It may be a coincidence, but this disease-fighting diet is also known to induce autophagy1,2,3.
Aerobic exercise
Aerobic exercise – exercise that requires oxygen to generate energy – is another way to increase autophagy1.
Good examples of aerobic exercise include walking, jogging, swimming and cycling.
We already knew exercise was good for our health. But research now suggests that many of these health benefits may be a direct result of autophagy activation:
“Exercise induced autophagy in multiple organs involved in metabolic regulation, such as muscle, liver, pancreas and adipose tissue. […] Physical exercise has numerous health benefits, such as life-span expansion, and protection against cardiovascular diseases, diabetes, cancer and neurodegenerative diseases. […] Many of these health benefits overlap with known protective functions of the cellular pathway of macroautophagy […] Thus, we proposed that some of the health benefits of exercise may be due to autophagy activation.”
Exercise induces autophagy in peripheral tissues and in the brain. PMCID: PMC3463459
Supplements
Many health supplements have been demonstrated to increase autophagy. These include:
There’s good news for fans of hot beverages too! Both coffee1 and green tea1 have been shown to help induce autophagy.
Summary: health benefits of autophagy
“More broadly, on the basis of this newly discovered link between exercise, autophagy and altered metabolism, we speculate that autophagy may represent a cellular mechanism by which exercise prolongs life and protects against cancer, cardiovascular disorders and inflammatory diseases.“
Despite only first being observed in the early 1960s, autophagy is one of the most ancient defense processes against invading pathogens.
As we’ve seen, autophagy plays a crucial role in regulating response to infection and inflammation. It helps destroy unwanted or harmful cellular components and allows for them to be used elsewhere.
Not only that, this recycling process helps maintain cellular health and promotes efficient energy generation.
It’s perhaps unsurprising, then, that autophagy is associated with a great many health benefits including reduced risk of cancer, diabetes and even a longer and healthier life.
Yes, almost same.